The Equilibrium Expression, Keq
The equilibrium constant, Keq, is a mathematical relationship between the concentrations of reactants and products in a system when they are at equilibrium at a certain temperature. Keq is temperature dependent but pressure and catalysts do not have an impact on the value of Keq.
The general expression for Keq can be written as:
![Rendered by QuickLaTeX.com { K }_{ eq }=\cfrac { \left[ products \right] }{ \left[ reactants \right] }](https://www.scienceflip.com.au/wp-content/ql-cache/quicklatex.com-39574e5d6b1328ba38247c1f793af049_l3.png)
When calculating Keq, both the concentration of species and the molar ratio from the chemical formulae are important. Consider the general reaction expression below:
![]()
The equilibrium constant, Keq would be written as follows:
![Rendered by QuickLaTeX.com { K }_{ eq }=\cfrac { \left[ { C }^{ c } \right] \left[ { D }^{ d } \right] }{ \left[ { A }^{ a } \right] \left[ { B }^{ b } \right] }](https://www.scienceflip.com.au/wp-content/ql-cache/quicklatex.com-4ba515f6a43fd1d2cbf04709495a21cc_l3.png)
Only species that have concentrations which can vary are included in the expression. Aqueous solutions and gases can have concentrations which vary so are included in the calculation of Keq. Solids and pure liquids are not included.
Homogenous Equilibrium
Homogenous equilibrium systems are ones where all substances are in the same phase. In homogenous systems where all species are in the same phase, solids and liquids are included in the calculation of the equilibrium expression, however, these systems are uncommon in this course.
It is common to see solids and pure liquids as part of a heterogenous equilibrium (different phases) and so are not included in the calculation of Keq in these instances.
Magnitude of Keq.
The value of Keq has no units but the magnitude of this value can tell us where the equilibrium position lies. That is, if there is a higher proportion of reactants or products present at equilibrium.
Keq has values between 0 and a large number generally up to the order of 105. The following are general assumptions we can make about the position of an equilibrium given the value of Keq:
- If Keq is close to 1 (0.1 – 10) there are relatively equal proportions of both reactants and products.
- If Keq is greater than 1 (10 – 10,000) products will be favoured in the equilibrium.
- If Keq is less than 1 (0.00001 – 0.1) reactants will be favoured in the equilibrium.
Keq for the Reverse Reaction.
As equilibrium systems involve both a forward and a reverse reaction, there also exists a value for Keq for each reaction. However, this value is different depending on the rate of reaction and the direction. Keq for the reverse reaction is simply the inverse Keq of the forward reaction:
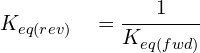
ICE Tables
ICE tables are a common tool which are used to solve equilibrium problems where all of the final equilibrium conditions may not be given. Usually, all of the initial concentrations are given or can be determined along with either the final concentration or the change in concentration of some of the products. This information can be used to determine the final concentrations of all species and this is applied to the equilibrium expression, Keq.
- I – initial concentration
- C – change in concentration
- E – equilibrium concentration
To determine the final equilibrium concentrations of all reactants and products, the changes in concentration are applied to the balanced equation.
See example 2 below:
Reaction Quotient, Q
The reaction quotient, Q, is used when a system is not at equilibrium and can help determine which way the system may move in order to achieve equilibrium. This will allow us to state if the production of reactants or products is favoured in order to reach equilibrium.
![Rendered by QuickLaTeX.com Q=\cfrac { \left[ { C }^{ c } \right] \left[ { D }^{ d } \right] }{ \left[ { A }^{ a } \right] \left[ { B }^{ b } \right] }](https://www.scienceflip.com.au/wp-content/ql-cache/quicklatex.com-d5c2fa7107aae1416c426b1610677477_l3.png)
Comparing Keq and Q
Comparing the values of Q to a stated value of Keq for an equilibrium system indicates which way the system may shift:
If Q < Keq, the equilibrium system will shift to the right to favour the forward reaction. This favours the formation of products over reactants.
If Q > Keq, the equilibrium system will shift to the left to favour the reverse reaction. This favours the formation of reactants over products.
Example 1:
Write the equilibrium expression for the following reaction: 2NO2(g) ⇌ N2O4(g)
Answer:
![Rendered by QuickLaTeX.com { K }_{ eq }=\cfrac { \left[ { N }_{ 2 }{ O }_{ 4 } \right] }{ \left[ { N }{ O }_{ 2 } \right] ^{ 2 } }](https://www.scienceflip.com.au/wp-content/ql-cache/quicklatex.com-cd184c1ba6025bccb3ae3dc7515f73cc_l3.png)
Example 2:
PCl5(g), PCl3(g) and Cl2(g) exist together in an equilibrium according to the following equation:
PCl5(g) ⇌ PCl3(g) + Cl2(g)
0.50 moles of PCl5(g) were placed into a 2L container. At equilibrium, there were 0.20 moles of PCl5(g) remaining. Complete an ICE table to determine the final concentrations of each component and use this to calculate Keq:
Answer:
| [PCl5(g)] | [PCl3(g)] | [Cl2(g)] | |
| Initial | 0.50/2 = 0.25M | 0 | 0 |
| Change | – 0.20/2 = 0.10 | + 0.10 | +0.10 |
| Equilibrium | 0.15 | 0.10 | 0.10 |
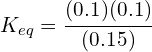
![]()
